Acute myeloid leukemia (AML) is a complex disease characterized by diverse molecular pathogenesis. Genetic alterations, including germline and somatic variants in the DEAD box helicase 41 gene ( DDX41) located on chromosome 5 play an increasingly recognized role. Recent reports indicate that 5% of intensively treated adult AML patients harbor DDX41 germline mutations ( DDX41MutGL), and their precise impact remains incompletely understood. These studies suggest that DDX41MutGL may define a distinct biological subgroup, associated with e.g. older age, male gender, low blast, and low white blood cell count (WBC).
To further elucidate the role of DDX41 in AML, we performed a retrospective analysis of 906 unselected adult AML patients from the AML Cooperative Group (AMLCG) registry (2015-2022), by targeted sequencing. As DDX41MutGL are typically “null mutations”, leading to reduced DDX41 expression, we additionally investigated whether DDX41 gene expression correlates or resembles the observed germline phenotype. Our analysis encompassed >1000 independent gene expression profiles (GSE37642, GSE14468, and GSE106291) of intensively treated adult AML patients. We identified a sub-cohort with low DDX41 ( DDX41-low) expression and re-analyzed them with next-generation sequencing to detect DDX41MutGL. Additionally, we correlated gene expression data with 198 DNA methylation profiles (from patients who had undergone both analyses) to identify potential epigenetic mechanisms underlying DDX41-low expression.
Among the 906 patients (median age 61 years; range 18-98 years), we identified 11 unrelated individuals with suspected DDX41MutGL (VAF > 40%). Notably, the overall frequency of DDX41MutGL in the German unselected AML patient population was merely 1%, considerably lower than previously reported (p<0.0001 in comparison to Duployez et al. 2022). Characteristically, DDX41MutGL patients in this cohort were mostly male (73%), with a median age of 67 years displaying low WBC (average 1,3G/l) and a normal karyotype (80%) at initial diagnosis.
Remarkably, our analysis of independent large gene expression cohorts revealed a pattern of phenotypic association in patients with decreased DDX41 expression ( DDX41-low) closely resembling those with DDX41MutGL. These associations were consistent across different datasets and included e.g. older age, low WBC, and low blast count. Furthermore, DDX41-low patients had poor overall survival. The DDX41-low subgroup constituted almost 10% of all AML patients, surpassing the reported frequencies of DDX41MutGL. In an exploratory pilot study, we screened 48 patients exhibiting the lowest DDX41 gene expression by sequencing the coding regions for DDX41 mutations but found none, suggesting alternative mechanisms, such as copy number changes, non-coding alterations or aberrant DNA methylation patterns that may replicate the phenotypic effects associated with DDX41MutGL.
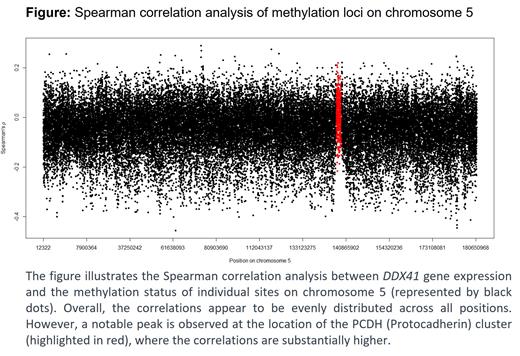
To further elucidate this mechanism, we correlated the gene expression of DDX41 with all DNA methylation loci (CpG sites covered by the EPIC array) on chromosome 5 using Spearman correlation analysis (GSE106291). We plotted the correlations against the sorted chromosome length, identifying a peak at a specific location corresponding to the PCDH (Protocadherin) cluster (Figure). Thus, DDX41-low was associated with reduced methylation at the PCDH loci and linked to higher PCDH gene expression. Across several data sets, we confirmed the association between higher PCDH cluster gene expression and low DDX41 expression.
In summary, our study reveals that the frequency of DDX41MutGL in an unselected population of German AML patients is considerably lower than previously reported. Additionally, we found that apart from DDX41MutGL, DDX41-low exhibits a comparable clinical profile and is linked to specific methylation and gene expression patterns, with a notable emphasis on the PCDH complex located on chromosome 5. Understanding the functional implications of PCDH genes and their interaction with DDX41 in AML may significantly advance our knowledge of AML pathogenesis. Gaining insights into DDX41 alterations may open avenues for personalized therapeutic approaches and further prognostic stratification for AML patients with distinct molecular characteristics.
Disclosures
Krug:AbbVie: Honoraria; Sanofi: Honoraria; Leo Pharma: Honoraria; BMS: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal